How is cellular diversity in the gut established and controlled?
Area 1: Transcriptional and Epigenetic Control of Intestinal Identity
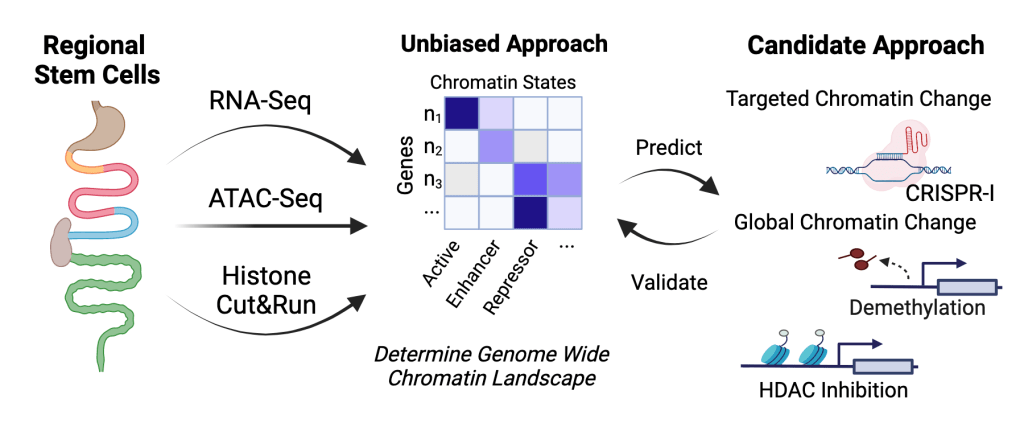
Adult intestinal stem cells continuously replace the epithelial lining of the intestine. When the stem cells are not able to replace the epithelium, devastating diseases including Inflammatory Bowel Disease and Cancer emerge. A major question in the field is how stem cells in different regions are programmed to give rise to regionally specific intestinal functions, and how that control is altered in disease. We believe that stem cells have heritable, regionally specific epigenetic states that govern the regional identities of their daughter differentiated cells. Our labs uses techniques from systems biology, genomics, and stem cell culture to profile multiple transcriptional, epigenetic, and chromatin accessibility traits in intestinal cells so that we can comprehensively define the chromatin landscape in multiple gut regions. We are identifying factors that control regional gut functions to find new ways to repair injured epithelium and treat metabolic diseases.
Area 2: Molecular Logic of Enteroendocrine Cell Diversity
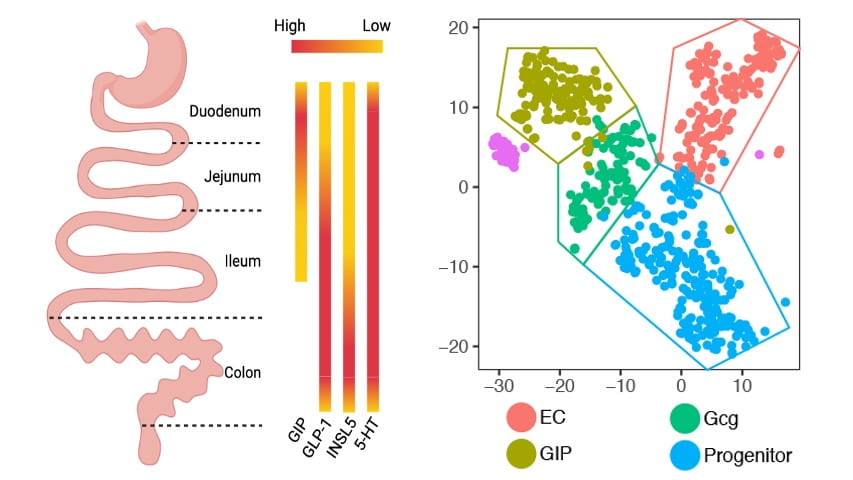
The largest endocrine organ in the body is the intestinal epithelium, where more than 20 different hormones are produced from rare Enteroendocrine cells. These hormones are critical regulators of hunger and metabolism, and new treatments derived from gut hormones are revolutionizing the treatment of obesity. But our understanding of the intestinal endocrine system lags far behind other endocrine organs, because these cells are rare, heterogenous, and hard to study using conventional methods. We have recently developed a novel cell culture system that allows us to model Enteroendocrine cell development in vitro from mouse and human intestinal stem cells. Using these tools, we are applying cutting edge genomic and single cell technologies to define the molecular logic of Enteroendocrine cell development.
Area 3: Inter-organ Cross-Talk that Controls Gut Function
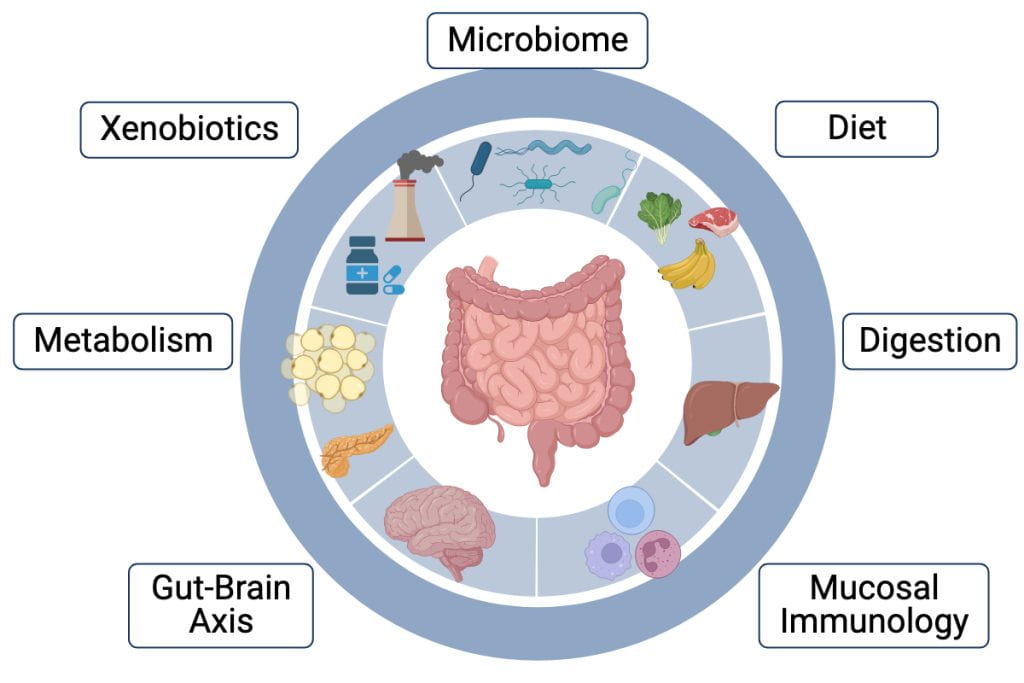
The gut integrates environmental, dietary, and microbial signals and coordinates a variety of host responses in the immune, nervous, and metabolic systems. We’re interested in understanding how signals from these systems modify intestinal cellular development and function, and in turn how secreted signals from the gut influence distant target organs. We have identified candidate inter-organ signaling networks and are using in vitro screens and animal models to determine the roles of these signals in health and disease